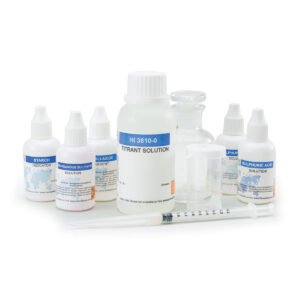
The HI3843 is a titration-based chemical test kit that determines the hypochlorite concentration within the 50 to 150 g/L Cl2 range. The HI3843 is supplied with all of the necessary reagents and equipment to perform the analysis. The test kit contains enough reagents for perform approximately 100 tests.
Features at-a-glance
Complete setup
- All required materials are included with the test kit, such as the Erlenmeyer flask, indicator and reagent bottles and packets, and plastic pipettes.
High resolution
- Readings from 50 to 150 g/L are determined to 5 g/L (0.5%) resolution.
Replacement reagents available
- There is no need to buy a new kit when reagents are exhausted. The HI3843-100 can be ordered to replace the reagents supplied with the kit.
Significance of Use
Hypochlorites are common bleaching agents used to whiten textiles and paper and to disinfect solutions. Sodium hypochlorite solution has been traditionally used for the treatment of pool water since it is an inexpensive and readily available form of chlorine. The solution usually contains 10 to 15% available chlorine (equivalent to 100 to 150 g/L), but it rapidly loses its strength during storage. In addition, since it is greatly affected by heat, light, pH, and heavy metals, it needs to be monitored regularly.
An iodometric titration method is used in the HI3843 test kit. The hypochlorite solution is treated with potassium iodide and strongly acidified with acid (Step 1). The amount of iodine generated is equivalent to the chlorine in the sample. The concentration of iodine is then calculated by titration of thiosulfate ions that reduce the iodine back to iodide ions (Step 2).
Step 1: OCl– + 2H+ + 2I– ? Cl– + I2 + H2O
Step 2: I2 + 2(S2O3)2- ? 2I– + (S4O6)2-
| Specification Name | Detail |
|---|---|
| SKU | HI3843 |
| Type | titration |
| Smallest Increment | 5 g/L (ppt) |
| Method | iodometric |
| Number of Tests | 100 avg |
| Ordering Information | HI3843 test kit comes with 30 mL potassium iodide solution, 100 packets bleach reagent B, 60 mL bleach reagent C (2), 125 mL glass Erlenmeyer flask and 1 mL plastic pipettes (25). |
| Reagent | HI3843-100 |
| Hypochlorite Range | 50-150 g/L (ppt) |
| Proposition 65 | WARNING: This product can expose you to chemicals including Ethylene glycol (ingested), which is known to the State of California to cause birth defects or other reproductive harm. |