Advantage:
1.Internal control: Human β-Globin gene as the internal control is included into the reagent to verify the validity of the experiment.
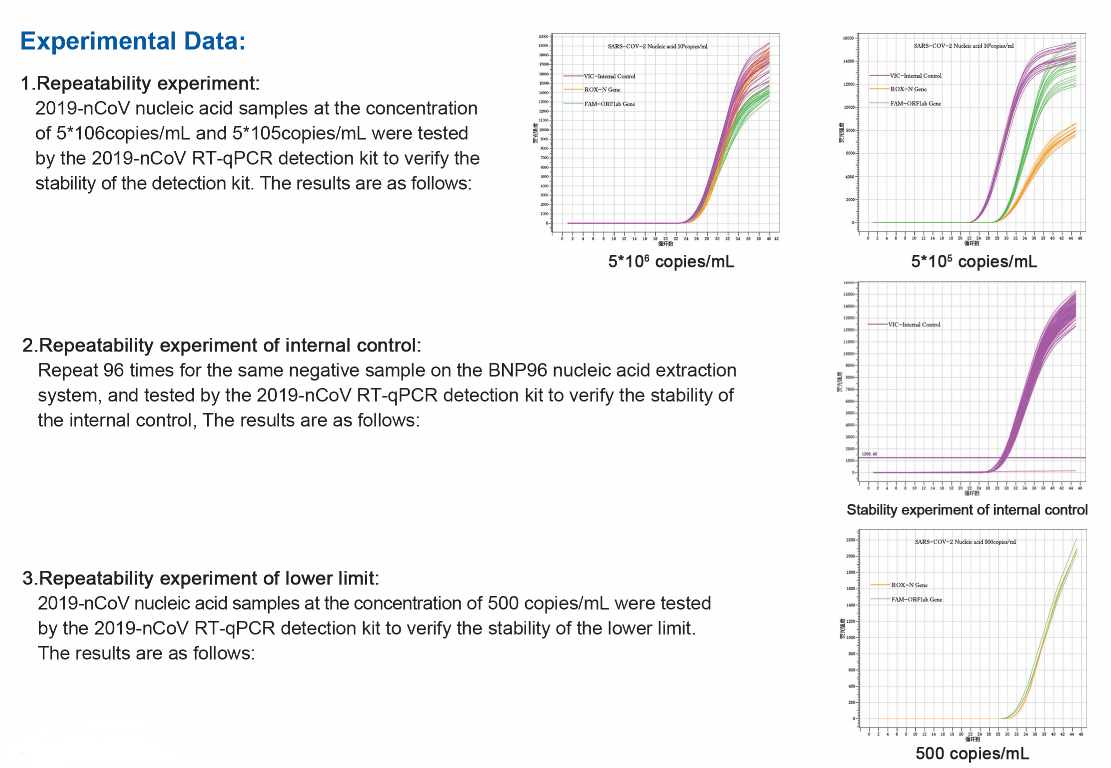
2.High sensitivity: The lowest detection limit is 500 copies/ml.
3.High specificity: Primers and probes are designed for specific fragments of two gene regions, which confirm each other to make the results more accurate. No cross-reactivity with other pathogens with the same site of infection or similar infection characteristics.
4.Strong stability: CV of each channel is all <3%.
5.Multiple Real-time RT-PCR detection: Each channel does not interfere with each other, and the amplification curve is S-shaped.
6.Simple operation: one-step method to complete RT-PCR, The whole procedure can be detected within 80min.
7.Fast speed: The PCR amplification time is less than 80 minutes.
Models:
|
Product Name |
2019-Novel Coronavirus (2019-nCoV) RT-QPCR Detection Kit |
|
Detection Principle |
Fluorescence PCR |
|
Detection Target |
Novel coronavirus (2019-Ncov) ORF1ab and N gene |
|
Sample Type |
Human oropharyngeal swabs, nasopharyngeal swabs and sputum |
|
Applicable Instrument |
Fluorescence quantitative PCR instrument |
|
Storage Conditions |
-20±5℃, keep away from light |
|
Valid Period |
Unopened ≥6 months; Opened≥90 days |
|
Sample Volume |
7μl |
|
Reaction Volume |
20μl |
|
Detection Limit |
500 copies/ml |
|
Stability |
CV <3% |
|
Interpretation of Positive Results |
CT≤38 |
|
Packing Specification |
48T/box; 60 boxes/carton |
|
Packet Size |
500*500*500mm |
|
Gross Weight |
23kg |
*Not Available in the U.s